GREASE THOSE WHEELS (CHEMISTRY)
#1 EQUILIBRIA
-------------------------------------------------------------------------------------------------------------------
Dynamic Equilibrium is a term used in chemistry that represents the state of a chemical reaction when both the forward and backward chemical reactions occur at corresponding or equal rates. For example a block of ice at 0* will both be melting and freezing but none of the processes seem to be happening. But if we start providing heat then the ice will melt visibly and vice versa. So we come to know that equilibrium can be affected by some physical changes So we get a more refined definition of dynamic equilibrium i.e when a chemical reaction can take place in both the direction at equal rates provided that physical conditions remain constant. Remember when a dynamic equilibrium is reached the concentration of reactants and products becomes constant (but never or very rarely equal) but the reaction never stops it is just continuing at the same rate on both sides. Also as the dynamic equilibrium is reached both products and reactants are present in the mixture.
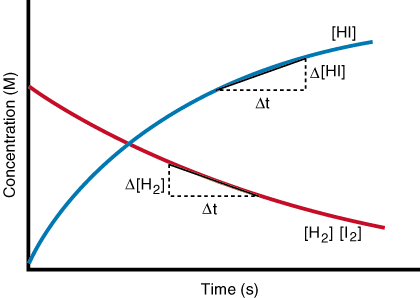
 The graph on the left side explains this phenomenon. At the beginning the concentration of reactants is higher so the number of effective collisions between the reactant molecules result in a faster initial rate of reaction but as their concentration drops and the concentration of products increases, the reverse reaction speeds up and the forwards reaction slows down eventually both the reaction rates balance out each other but the concentration is never the same for products and reactants but constant.
The graph on the left side explains this phenomenon. At the beginning the concentration of reactants is higher so the number of effective collisions between the reactant molecules result in a faster initial rate of reaction but as their concentration drops and the concentration of products increases, the reverse reaction speeds up and the forwards reaction slows down eventually both the reaction rates balance out each other but the concentration is never the same for products and reactants but constant.
Remember in exams you might probably be given a concentration/time graph. The thing is same except that it would be more like a cross. (see above)
-------------------------------------------------------------------------------------------------------------------
Another important part of this chapter are the dreaded calculations involved. The Equilibrium Constant is the constant value (K) of a chemical reaction at an equilibrium where Kc equals... [c denotes the concentrations i.e we are talking about solutes right now, no pure solids or stuff 'ere]
-------------------------------------------------------------------------------------------------------------------
Now lets get to the interesting Le Chatlier's Principle.
Le Chatlier's principle is probably the easiest and most enjoyable part of your chemistry course, if only you find the way to enjoy it.
Basically Le Chatlier was a philosopher who proposed this theory in the 19th century [you might be asking what philosophers have to do with chemistry, but heres a hint for those who don't believe me!! This stuff really is easy...=)]
Consider a chemical reaction in equilibrium as a despotic, old lady who is half-asleep and can shrink or expand and hates strong smells or scents!! and yourself as a mischievous little boy who has only one thing on his mind...the unrest of the old lady!! (nice story eh?)
You have a menu of disturbances which is filled with things you can do to disturb the old lady...heres that menu...
- explain, in terms of rates of the forward and reverse reactions, what is meant by a reversible reaction and dynamic equilibrium
- state Le Chatelier's Principle and apply it to deduce qualitatively (from appropriate information) the effects of changes in temperature, concentration or pressure, on a system at equilibrium
- deduce whether changes in concentration, pressure or temperature or the presence of a catalyst affect the value of the equilibrium constant for a reaction
- deduce expressions for equilibrium constants in terms of concentrations, K c , and partial pressures, K p [treatment of the relationship between K p and K c is not required]
- calculate the values of equilibrium constants in terms of concentrations or partial pressures from appropriate data
- calculate the quantities present at equilibrium, given appropriate data (such calculations will not require the solving of quadratic equations)
- describe and explain the conditions used in the Haber process and the Contact process, as examples of the importance of an understanding of chemical equilibrium in the chemical industry.
Equilibria remains as one of the most daunting tasks for students of AS Level Chemistry, but with the clearance of some simple concepts, this topic becomes a piece of cake!!
A reversible reaction is the a chemical reaction that can proceed in both forward and backward directions, depending upon the conditions.
For e.g.. CaCO3 >>><<< Ca + CO2
Dynamic Equilibrium is a term used in chemistry that represents the state of a chemical reaction when both the forward and backward chemical reactions occur at corresponding or equal rates. For example a block of ice at 0* will both be melting and freezing but none of the processes seem to be happening. But if we start providing heat then the ice will melt visibly and vice versa. So we come to know that equilibrium can be affected by some physical changes So we get a more refined definition of dynamic equilibrium i.e when a chemical reaction can take place in both the direction at equal rates provided that physical conditions remain constant. Remember when a dynamic equilibrium is reached the concentration of reactants and products becomes constant (but never or very rarely equal) but the reaction never stops it is just continuing at the same rate on both sides. Also as the dynamic equilibrium is reached both products and reactants are present in the mixture.
 The graph on the left side explains this phenomenon. At the beginning the concentration of reactants is higher so the number of effective collisions between the reactant molecules result in a faster initial rate of reaction but as their concentration drops and the concentration of products increases, the reverse reaction speeds up and the forwards reaction slows down eventually both the reaction rates balance out each other but the concentration is never the same for products and reactants but constant.
The graph on the left side explains this phenomenon. At the beginning the concentration of reactants is higher so the number of effective collisions between the reactant molecules result in a faster initial rate of reaction but as their concentration drops and the concentration of products increases, the reverse reaction speeds up and the forwards reaction slows down eventually both the reaction rates balance out each other but the concentration is never the same for products and reactants but constant.Remember in exams you might probably be given a concentration/time graph. The thing is same except that it would be more like a cross. (see above)
Another important part of this chapter are the dreaded calculations involved. The Equilibrium Constant is the constant value (K) of a chemical reaction at an equilibrium where Kc equals... [c denotes the concentrations i.e we are talking about solutes right now, no pure solids or stuff 'ere]
[C]^c x [D]^d
---------------
[A]^a x [B]^b
Confusing? Lets see it in another way! aA + bB >>><<< cC + dD, so we come to know that...
- The formula in its most basic form is products over reactants.
- where the number of moles of the products and reactants are their specific powers in the formula [the smaller alphabets, i could not format the article to show them as powers]
- the enclosing square brackets, i.e, '[]' denote the equilibrium concentration in mol/dm3 of the compound/element they enclose.
- no terms related to pure solids or liquids like, water should be included in our calculations!
- When the question requires the units of Kc then they are given by the following formula, mol/dm3^(c+d)-(a+b).
- We should always follow what the question says when N2 + 3H2>>><<<2NH3 is written like this we take NH3 to be the product but when it is written like 2NH3>>><<
2 + 3H2 we take NH3 as the reactant. Don't forget that!!! If the number of moles on both sides are equal then we can use the number of moles instead of concentration while calculating. When you are given initial concentrations of the reactants and are required to find out the equilibrium concentrations of both the reactants and the products then we used the algebraic x stuff. For example for this equation (0.2 mols (initial) of H2S are given) 2H2S(g)>>><<< 2H2(g) S2(g) x stuff will be as follows...Hydrogen Sulfide use 2x (0.2-2x), Hydrogen gains 2x (0+2x) and Sulfer gets x only (0+x) we then put these concentrations in the formula to get the value of x and subsequently find their equilibrium concentrations. - If the equilibrium constant is very large than we assume that very little of one of the reactants has remained unreacted. On the contrary if the equilibrium constant is very small then we assume that very little of the products is produced.
Remember the value of Kc only depends upon Temperature and nothing else. Some commonly mistaken effectors include.
- The initial concentration of the reactants or products.
- The direction from which equilibrium is approached either via decomposition or composition.
- Pressure (in the case of gaseous equilibria only).
- Usage of a catalyst.
- For a reaction that is exothermic in the forward direction, the value of Kc decreases with an increase in temperature and vice versa as more of the endothermic reactants produced to annul this change. [Remember Kc is all about products over reactants, if we increase the divisor and decrease the dividend the answer gets smaller each time]
- And for reactions that are endothermic in the forward direction , the value of Kc increases with an increase in temperature and vice versa as more of the endothermic products produced to annihilate this change.
- The usage of a catalyst allows equilibrium to be maintained in less amount of time as the catalyst speeds up both forward and backward reactions equally by lowering the activation energy of the reaction.
Now lets talk about gases, i.e, gaseous equilibrium constant or Kp. gases get separate calculations because it is more convenient to use their partial pressures (the individual pressure of a gas if it were to occupy whole space at room temperature; Remember: the partial pressure of a gas is directly proportional to its concentration or mole fraction, i.e moles of gas/total moles in a mixture) instead of concentrations.
This is almost the same stuff as the Kc except that you never ever use square brackets instead you stick the partial pressure of each gas with it in the form of a multiplier.=P this stuff is getting on my nerves!!!
And yes you get the partial pressure of a gas by the following relationship: p= total pressure of the gas mixture (atm or kPa) x the mole fraction of the gas involved.
Thank the Almighty that we are over with this part!!!
Now lets get to the interesting Le Chatlier's Principle.
Le Chatlier's principle is probably the easiest and most enjoyable part of your chemistry course, if only you find the way to enjoy it.
Basically Le Chatlier was a philosopher who proposed this theory in the 19th century [you might be asking what philosophers have to do with chemistry, but heres a hint for those who don't believe me!! This stuff really is easy...=)]
".. states that when stress (change in physical condition[s]) is applied to a system (chemical reaction) in equilibrium, then the system responds (changes in the concentrations of reactants or products) in such a way so as to cancel out the some of the effect of the applied stress."The following hypothesis will really help...
Consider a chemical reaction in equilibrium as a despotic, old lady who is half-asleep and can shrink or expand and hates strong smells or scents!! and yourself as a mischievous little boy who has only one thing on his mind...the unrest of the old lady!! (nice story eh?)
You have a menu of disturbances which is filled with things you can do to disturb the old lady...heres that menu...
- Increasing the temperature by turning on the room heater.
- Decreasing the temperature by switching on the air conditioner.
- Increasing the pressure by closing all windows and ventilators.
- Decreasing the pressure by opening all windows and ventilators.
- Increasing the concentration of reactant by throwing in a bucket of your socks that have not seen the laundry for a month. (strong smell)
- Increasing the concentration of product by spraying a lot of air freshener. (strong scent this time)
And the results would be like...
- She turns off the heater and switches on the air conditioner.
- She switches off the air conditioner and turns on the heater.
- She Shrinks [!] so as to occupy less space.
- She Expands [!] so as to occupy more space.
- She sprays air freshener all around her.
- She borrows your socks [!].
Now lets see all of this in a scientific context. But before we move on, you should know these important points...
- Every equilibrium reaction is either exothermic (giving off heat) or endothermic (absorbing heat) in the forward direction and in the backwards proceeding direction its energy profile is opposite to that of the forward reaction. For example, if the forward reaction is exothermic then the reverse reaction will be endothermic.
- Changes in pressure refer to the changes in partial pressures of gases taking part in reaction and doesn't include gases which are inert or do not take part in any reaction with the already present elements or compounds.
- Partial pressures of gases are changed by changing the volume of the vessel \ container the reaction is taking place in.
- Equilibrium is always approached from two directions both the forward and reverse reaction should equal each other's rates for equilibrium to establish.
- The response of the system when subjected to stress will act to only relieve its effect and not fully cancel it out. For example if a reaction involving hydrogen gas is at equilibrium with 1 mol of H2 present in the mixture, we apply stress by removing 0.3 moles of the gas from the container and so the system (the reaction) tries to relieve some of the stress by producing more H2 gas but it will not fully compensate for the loss so the amount of moles present in the new equilibrium mixture will always be greater than 0.7 moles and less than 1.0 mole. Think of it in the old lady-rude boy's context (stated above) that the mischievous boy leaves his smelly socks in the old ladies room thereby reducing the affect of her air freshener, so she in retaliation sprays some more air freshener but still the some of the smell remains in the room.
Moving on we will use the following reaction to link to the old lady-rude boy hypothesis above...
3H2 + N2 >>><<< 2NH3 (the reaction is exothermic (gives off heat) in the forward direction)
- Increasing the temperature; the reverse reaction will come into play as it is endothermic in nature and the Air conditioner is turned on (the rate of reverse reaction will increase) to reduce the applied stress. As a result more hydrogen and nitrogen will be produced and their increased concentration would lead to increased successful collisions between their molecules and so more ammonia will be produced, eventually both the forward and reverse reactions balance out and a new equilibrium is established.
- Decreasing the temperature; the rate of forward will increase as it is exothermic in nature. The heater of the reaction is turned on to combat the decrease in temperature. The mechanism of approaching equilibrium is the same as above.
- Increasing the pressure (partial pressure); the reaction 'shrinks' to occupy less space. Look at the product and reactant sides and think of moles as the number of citizens of a country that are taking part in a reaction. As I told you above that partial pressure of gases can only be changed by altering the volume of the container so as to increase the p.p of gases you have to decrease the volume of the container, a decreased container volume means that to relieve the system of such stress the equilibrium reaction has to shift towards the side with the lesser number of citizens in it in the above mentioned reaction the product side has 3 citizens from hydrogen republic and a sole representative from the peoples republic of nitrogen, adding up to 4 people, while the reactant side features only 2 people from the Kingdom of Ammonia thus the reaction favours the side with the least number of people when the size of container is decreased and thus the rate of forward reaction will increase.
- Decreasing of pressure (partial pressure); the reaction expands to occupy more space. This time around the reverse reaction is favoured as the system wants more citizens to be present in the container to relieve it of the stress that is there due to a sudden decrease in space. Think of a chemical reaction as the sleeping old women...it doesn't like to get disturbed!!
- Increasing the concentration of reactants; Increasing the concentration of reactants would result in the equilibrium being disturbed so the system wants to get close to its comfort or equilibrium level and thus starts to use up these additional reactants and the equilibrium shifts towards the forward direction. Much like when smelly socks (reactant) are introduced into the old woman\s fresh smelling room, she strives to get close to her comfort level by spraying more air freshener to reduce the smell (product).
- Decreasing the concentration of reactants; This time around the result will be opposite to what I have told you above.
Thats it folks...do comment and let me know about what you think about his...=)

1 comment:
good one! really was a help to me especially that Le Chatlier part...gg Creative!!
Post a Comment